Roughly 10 million people fell ill with TB in the last year and 1.6 million people were killed by the disease. While significant advances have been made in detecting and treating TB, drug-resistant strains threaten to derail progress. For the half million people who contract multidrug-resistant TB (MDR-TB) each year, the treatment regimen is a demanding course of medicines that can cause serious side effects, including hearing loss and renal impairment. STREAM, the largest recruited multi-country clinical trial to examine shortened regimens for MDR-TB, aims to generate the highest-quality evidence regarding the efficacy, safety, and cost of shorter, more tolerable MDR-TB regimens.
This month, Vital Strategies and its TREAT TB partners participated in the 50th Union World Conference on Lung Health in Hyderabad, India. We’ve curated videos, papers, and blogs related to this event and the work conducted by Vital Strategies’ Research Division.
Latest From the Research Division and Treat TB Blog
Promising Developments for Better Treatments
Gay Bronson, Deputy Director, Research Division, discusses progress in the treatment of multi drug-resistant tuberculosis in this recent Q&A.
More from the blog:
Highlights from The Union’s 50th World Conference on Lung Health
What To Watch
Why Community Engagement Matters
Community engagement throughout the research cycle leads to awareness, involvement, trust and, ultimately, contributes to better outcomes. Learn the fundamentals from this video.
Expert Focus
The results from Stage 1 of the landmark STREAM clinical trial were published in the New England Journal of Medicine in March 2019, supporting the move to shorter, more cost-effective treatment regimens for multi-drug resistant tuberculosis. Commenting on the work, Dr. I.D. Rusen, Vital Strategies Senior Vice President, Research and Development and Director of the USAID-funded TREAT TB project, which is implementing STREAM, said, “Until now there has been a lack of strong supporting evidence to underpin MDR-TB treatment guidelines. The results from STREAM Stage 1 help to fill that gap. The final results show that the trial setting meant more patients successfully completed treatment on the 20–24-month regimen than we know is often the case in real life settings. In routine programs unable to achieve the high STREAM retention rates, the 9–11-month regimen may actually perform better in comparison to the longer regimen.” Read more at treattb.org.
From The Field
Unfinished Business
While the results of STREAM Stage 1 provided important evidence to support shorter regimens, read about why it’s imperative to complete Stage 2 of the trial in an interview with Dr. Daniel Meressa from St. Peter’s Tuberculosis Specialized Hospital in Addis Ababa, Ethiopia.
Community Engagement: A Two-Way Street
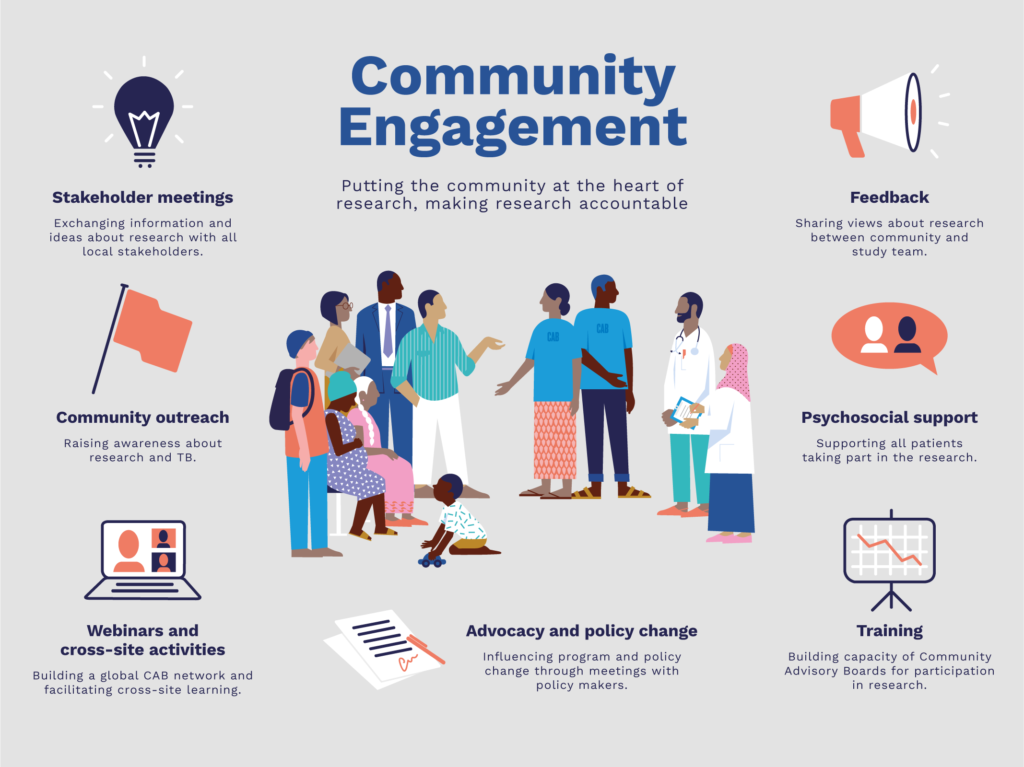
Community advisory boards (CABs) provide crucial feedback from STREAM participants and their families to researchers and other trial implementers. They also play an important role in raising awareness of TB and the trial, and make complex information accessible to affected communities. The psychosocial support they provide to trial participants and family members can help improve retention and adherence rates for clinical trials. Sister Mary Josephinal Francis, STREAM CAB Coordinator in Chennai, India, discusses the importance of this holistic approach in this blog.
Making History in Mongolia
In September 2014, the first patient was recruited to the STREAM trial in Mongolia, one of the countries in the Western Pacific Region identified by the WHO as having a high TB burden. Watch participants and clinicians discuss the important work carried out in that country.
Global Policy
TB is the world’s most deadly infectious disease, killing 1.6 million people a year—despite being both preventable and curable. Read José Luis Castro’s call to action from World TB Day.
Resources and Research
A Trial of a Shorter Regimen for Rifampin-Resistant Tuberculosis
WHO Global tuberculosis report 2019
Treat TB Operational Research E-learning Program